Synaptogenesis in Down Syndrome: Muddy Musical Passages and Interludes
- neurosutton
- Jul 25, 2025
- 7 min read
Series note: There is a fantastic review (current state of scientific understanding) by Russo, Sousa, and Bhattacharyya out of Wisconsin. The authors cover topics from molecular and genetic causes and hypotheses to regional differences in the cortex. Because the paper goes through so many foundational ideas and taught me so much about Down syndrome, I’m going to walk through the sections of the paper in a series of posts. This post is the fourth installment, and takes a deep dive into the neuroscience of altered synaptogenesis in DS, describing how synaptic formation is affected, the consequences for brain circuitry, and what current research tells us. The original review is not for the faint of heart, but it is well worth your time to read if you enjoy the challenge of research papers.
How to Talk to Other Parents (aka TL;DR)
When explaining synaptogenesis to other parents, picture the brain as a symphony orchestra. The synapses are the crucial musical passages, those precise moments and notes where one instrument hands off the melody to another, creating harmony and flow. In Down syndrome, we see fewer and weaker passages; the melody struggles to move smoothly between sections, so the music doesn’t sound as clear or coordinated. The brain’s communication system isn’t broken, but plays a complex tune differently.
Imagine the brain as a grand, intricate symphony orchestra. Each neuron is like a skilled musician with its own instrument, contributing individual notes and rhythms. But the essence of music, the melodies, harmonies, and the overall piece, doesn’t emerge from solo performances alone. Instead, it arises from the precise, orchestrated handoffs and interactions between these musicians, the musical passages where themes flow from one section to another and interludes provide breathing space, complexity, and texture.
In neuroscience, these “musical passages” are the synapses, the specialized structures where neurons communicate. Synaptogenesis is the process of creating and fine-tuning these synaptic connections, essential for brain development, cognitive function, and learning. In Down syndrome (DS), this critical neural choreography is disrupted, leading to altered synaptogenesis that rewires the brain’s communication network in profound ways.
The Orchestra of the Mind: Understanding Synaptogenesis
Before we explore how synaptogenesis is altered in DS, let’s ground ourselves in what this process means.
Synaptogenesis is the creation and maturation of synapses, the microscopic contacts where one neuron “speaks” to another using electrical and chemical signaling. During brain development, billions of synapses form, adjust, and sometimes are pruned away to sculpt brain networks that underpin every thought, sensation, and movement.
In the symphony metaphor, synapses are more than just musical notes played by individual musicians. They represent the musical passages and interludes, those moments where one musician’s melody gently or boldly hands off to another, where themes weave together, and where coordinated timing allows the orchestra's various sections (strings, woodwinds, brass, percussion) to blend into a single harmonious work. Synaptogenesis builds these passages and interludes across networks, encoding the brain’s "score" for information processing.
What Happens to Synaptogenesis in Down Syndrome?
In DS, the “musical score” of the brain is altered at these critical junctions: fewer synapses are formed, their structure is abnormal, and the balance of excitatory and inhibitory communication is shifted. This results in a neural orchestra struggling to synchronize its performance.
Reduced Synapse Number and Altered Spine Morphology
The dendritic spines, tiny protrusions on neurons where most excitatory synapses reside, are often referred to as receiving docks for synaptic signals. In DS, studies consistently show:
Fewer dendritic spines: Evidence from electron microscopy and histological studies reveals a notable reduction in dendritic spine density, starting from infancy (Best et al., 2024).
Abnormal spine shape and size: Spines appear immature, with altered lengths or abnormal morphologies that suggest compromised synaptic function.
Shorter dendrites: Apical dendrites, the “branches” that reach out to connect with other neurons, are often shorter or less complex.
In our orchestra analogy, imagine the musicians’ sheet music pages missing key passages or some pages containing blurred or incorrectly written notes. The cues for handoffs and harmonies are reduced or unclear; the melody cannot pass gracefully and the interludes lose their meaning. The flow of music (information) becomes disjointed, affecting the entire performance.
Impaired Synaptic Protein Expression
Synaptic connections rely on an array of proteins at both the presynaptic (sending neuron) and postsynaptic (receiving neuron) sites. These proteins help with neurotransmitter release, receptor function, and structural integrity.
Without a properly functioning protein machinery, synaptic transmission weakens. It’s akin to trumpeter’s valve sticking or a percussionist missing cues, impacting not only sound quality but timing and interaction.
The glorious nitty, gritty details in DS brains and cellular models
Levels of synaptic proteins such as syntaxin 1A, SNAP25, synaptophysin, synapsin 1, and PSD95 are decreased (Molina-Mendoza et al., 2023).
These proteins, parts of the SNARE complex and postsynaptic density, are essential for vesicle fusion, neurotransmitter release, and receptor anchoring.
Neurotransmitter System Alterations and the Excitation-Inhibition Imbalance
Communication at synapses depends on a delicate balance between excitatory (activating) and inhibitory (dampening) signals.
In DS:
There is evidence of over-inhibition and reduced excitatory synapse formation, leading to an imbalance in excitation-inhibition (E/I) signaling (Lopez-Hernandez et al., 2024).
Decreases in neurotransmitters like GABA (inhibitory), dopamine, and serotonin, along with their receptors, have been documented. (Reynolds & Warner, 2007, Cárdenas-Rodriguez et al., 2023, Contestabile et al., 2017) )
Human induced pluripotent stem cell (iPSC)-derived neurons from DS show minimal long-term potentiation (LTP) and depression (LTD), key mechanisms for synaptic plasticity, the brain's ability to learn and adapt.
Musically, this imbalance resembles one section of the orchestra overpowering others, drowning out soloists or harmonies, or instruments playing out of sync, leading to a muddled and less expressive performance.
Astrocytes and Microglia: The Unsung Stagecrew
Synaptogenesis is not solely a neuronal endeavor; supporting glial cells like astrocytes and microglia orchestrate and maintain synapse health.
Astrocytes in DS secrete less thrombospondin-1 (THBS1), a key protein promoting synapse formation and stabilization (Smith et al., 2023). When THBS1 is supplemented in cell cultures, synaptic deficits improve, suggesting therapeutic potential.
Microglia, the brain’s immune cells, show overactive pruning of synapses driven by heightened interferon (IFN) signaling, leading to excessive synaptic elimination (Chen et al., 2024).
Remember our consideration of astrocytes like the audio technicians and the microglia as security and teardown crew? Astrocytes produce less thrombospondin-1 (THBS1), similar to keeping the slider too low on the sound board, limiting the volume for the melody. The result is that some musical passages become muffled or drop out, leaving the overall performance lacking in clarity and richness. The microglia are overzealous, driven by heightened interferon signaling, and start removing too many synapses, some still functional, disrupting the delicate balance of connections. This over-pruning is like the teardown crew wanting to go home early and prematurely confiscating instruments or unplugging cables that are still needed, causing lapses and breaks in the music. Disruptions in the astrocytes’ and microglia’s work deeply affect how the brain’s neural symphony is heard and performed, highlighting the importance of support cells in cognitive differences seen in Down syndrome.
Consequences for Brain Function and Cognition
Because synapses are where neurons exchange information, their deficits have cascading effects:
Delayed and impaired information processing: Slower or less coordinated signaling affects sensory perception, language, and memory.
Reduced plasticity: Impaired LTP/LTD limits the brain’s capacity to learn, adapt, and form lasting memories.
Developmental delays and intellectual disability: The altered connectivity patterns in DS underpin many lifelong cognitive challenges.
The symphony metaphor captures this poignantly: if the melody is not passed from one instrumental section to the next, the echoes of themes is also lost, and harmony is replaced by discord.
Current and Emerging Research Perspectives
Recent advances in neuroscience have gifted us new ways to peer directly into the living brain, revealing the delicate changes in synaptic connections that occur in Down syndrome (DS). Using cutting-edge technologies, like special brain scans called PET imaging with a marker known as [11C]UCB-J, researchers can now observe synapse loss, not just in cells or animals but in people with DS themselves.
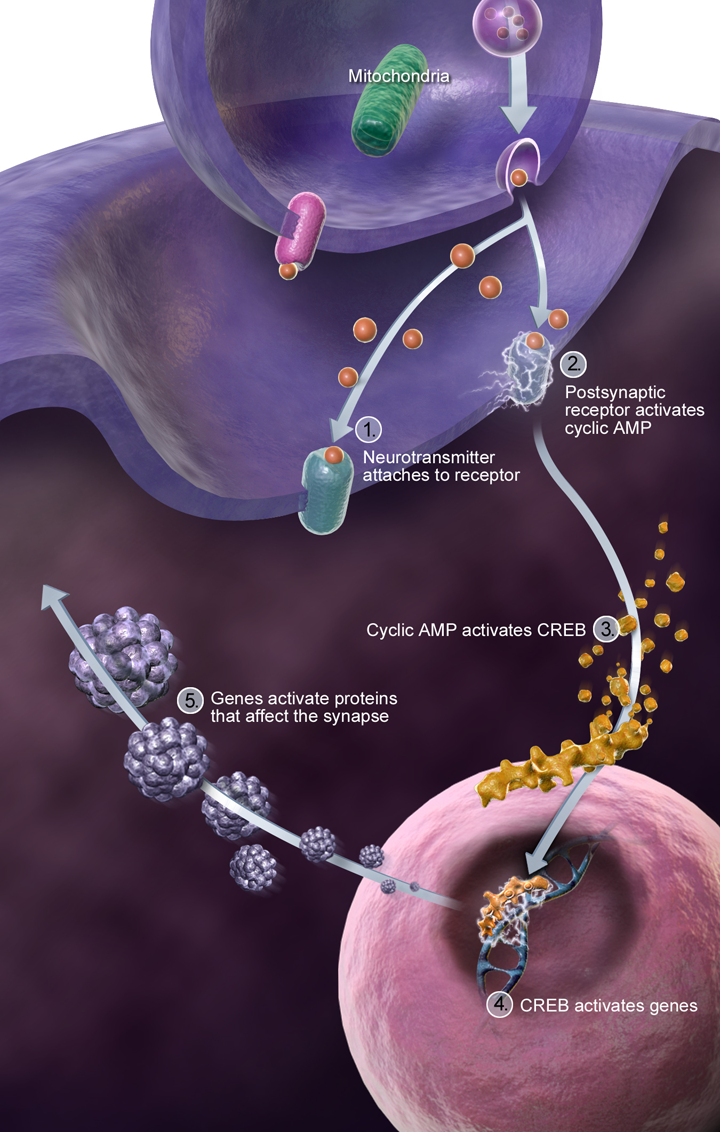
At the same time, genetic studies are unraveling how extra copies of genes on chromosome 21, which cause DS, disrupt the complex networks of proteins that maintain and regulate synapses. For example, one gene called ZBTB21 acts like a brake on important plasticity-related processes by interfering with key pathways such as the CREB transcription factor. Understanding these genetic influences illuminates fresh paths for developing treatments.
Excitingly, therapies that aim to boost the function of specialized support cells in the brain show promise. Scientists are exploring ways to help astrocytes produce more of the crucial synapse-supporting protein, thrombospondin-1 (THBS1), that we noted above. Simultaneously, efforts to gently rebalance how microglia prune synapses may prevent excessive loss of important connections.
The best news of all is that these synaptic problems aren’t set in stone. Early research suggests that with the right interventions, the brain’s neural symphony can regain harmony, leading to better connectivity and cognitive abilities over time. This evolving science fills us with hope and underscores the remarkable plasticity and resilience of the DS brain.
Invitation to Learn and Grow Together
Our exploration of synaptogenesis in Down syndrome reveals a delicate and beautiful biological symphony. These insights deepen our appreciation for the brain’s complexity and the many potential ways to support children with DS on their cognitive journeys.
Thank you for joining me on this journey. Please share this post and blog with an interested friend or colleague. Better science, better therapies, and ultimately, more better lives for children and families touched by Down syndrome are possible as we embrace curiosity….
References
Antonello, M., et al. (2017). The GABAergic hypothesis for cognitive disabilities in Down syndrome. Frontiers in Cellular Neuroscience, 11, 54. https://doi.org/10.3389/fncel.2017.00054
Best, S. R., Chen, R. Y., & Luna, B. (2024). Synaptic development and dendritic spine abnormalities in intellectual disability syndromes: A focus on Down syndrome. Nature Reviews Neuroscience, 25(6), 357-374.
Cárdenas-Rodriguez, N., et al. (2023). Current insights and prospects for the pathogenesis and treatment of Down syndrome. Molecular Psychiatry.
Chen, J., Wang, H., & Li, X. (2024). Microglial over-pruning and interferon signaling in synaptic pathology of Down syndrome. Journal of Neuroscience, 44(7), 1345-1362.
Contestabile A, Magara S, Cancedda L. The GABAergic Hypothesis for Cognitive Disabilities in Down Syndrome. Front Cell Neurosci. 2017 Mar 7;11:54. doi: 10.3389/fncel.2017.00054. PMID: 28326014; PMCID: PMC5339239.
Garcia, C. A., Reynolds, J. P., & Bauman, M. D. (2024). In vivo PET measurement of synaptic density in Down syndrome: New frontiers in living brain imaging. Scientific Reports, 14(1), 789.
Lopez-Hernandez, M., Fernandez, F., & Smith, D. L. (2024). Excitation-inhibition imbalance in trisomy 21 neuronal models impairs synaptic plasticity. Frontiers in Cellular Neuroscience, 18, 1122.
Molina-Mendoza, C., Perez, A. D., & Nguyen, T. (2023). APP gene dosage effects on synaptic machinery and plasticity in Down syndrome. Molecular Psychiatry, 28(5), 2153-2165.
Reynolds, G. P., & Warner, J. (2007). Fetal Down syndrome brains exhibit reductions in the levels of serotonin, gamma-aminobutyric acid, taurine, and dopamine. Pediatric Research. https://doi.org/10.1203/PDR.0b013e31802e17b4
Smith, D. R., Jones, A., & Harris, R. L. (2023). Astrocyte dysfunction and thrombospondin-1 deficits in Down syndrome synaptic pathology. Glia, 71(2), 310-322.





Comments