Understanding Down Syndrome through the Lens of Neuroscience: Genome-Wide Dysregulation, Epigenetics, Inflammation, and Metabolism
- neurosutton
- Jul 29, 2025
- 6 min read

Series note: There is a fantastic review (current state of scientific understanding) by Russo, Sousa, and Bhattacharyya out of Wisconsin. The authors cover topics from molecular and genetic causes and hypotheses to regional differences in the cortex. Because the paper goes through so many foundational ideas and taught me so much about Down syndrome, I’m going to walk through the sections of the paper in a series of posts. This post is the final installment, which looks at the molecular effects of an extra chromosome 21. The original review is not for the faint of heart, but it is well worth your time to read if you enjoy the challenge of research papers.
How to Talk to Other Parents (aka TL;DR)
Down syndrome affects not just chromosome 21 but impacts gene expression across the entire genome, alters how genes are epigenetically regulated and spatially organized, causes long-lasting brain inflammation, and disrupts cellular metabolism. These interconnected changes influence brain development and function, but new therapies targeting these areas offer hope for improved cognition and health.
Down syndrome (DS) is caused by an extra copy of chromosome 21, but its effects ripple far beyond that single chromosome. Groundbreaking research now reveals that DS profoundly alters gene expression across the whole genome, reshapes epigenetic regulation, fuels chronic inflammation in the brain, and disrupts cellular metabolism. Each of these interconnected processes shapes brain development, synapse formation, and neural signaling, offering multiple promising therapeutic targets.
Down Syndrome Is Much More than an Extra Chromosome
The extra chromosome sets off complex changes in how genes are expressed throughout the genome, not just on chromosome 21. Key pathways important for brain growth, like Wnt signaling and synapse formation, are disrupted, causing widespread molecular imbalance. Novel computational tools help identify critical genes to target therapeutically, aiming to restore balanced gene networks and improve brain function.
The glorious nitty, gritty details on key disrupted pathways
Wnt signaling: This pathway plays a pivotal role in embryonic brain development, helping regulate neural progenitor proliferation and differentiation. Altered Wnt signaling has been linked to abnormal brain structure and impaired synapse maturation in DS (Russo et al., 2023).
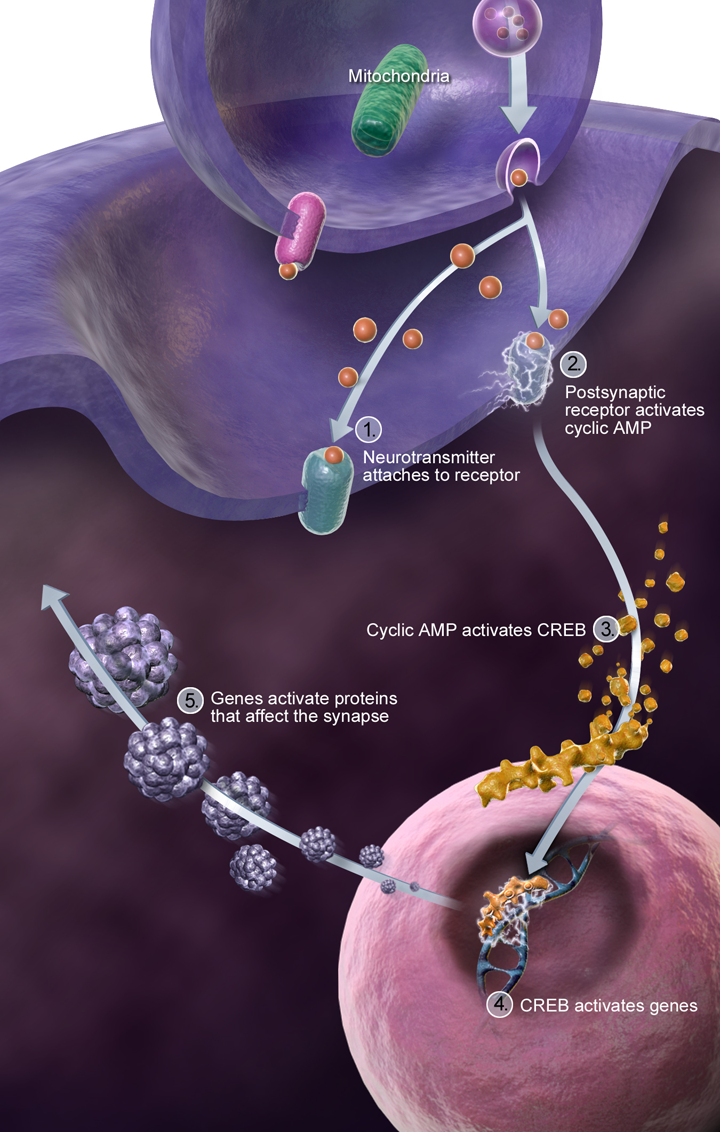
Synapse maturation: Synapses are the critical communication points between neurons. Changes in gene networks governing synaptic proteins disrupt how efficiently neurons connect and communicate, underlying many of the cognitive challenges in DS.
Genome-wide transcriptional dysregulation: Beyond chromosome 21, genes across other chromosomes also show altered expression levels, mediated by complex regulatory networks. Modern computational approaches, including machine learning, now help identify these critical “hub” genes, pinpointing potential therapeutic targets (Russo, 2023).
The Epigenome and 3D Genome Architecture: Brain’s Spatial and Functional Blueprint
Beyond the DNA code itself lies epigenetics, chemical modifications that dictate how and when genes turn on or off. These include DNA methylation, histone modifications, and chromatin accessibility. In DS neural progenitor cells, striking abnormalities in these marks have been documented.
Importantly, our genome is not just a linear string; it folds intricately in three-dimensional space inside the nucleus. This folding creates Topologically Associating Domains (TADs), which compartmentalize the genome to regulate gene expression programs precisely during development. Recent studies show that trisomy 21 triggers substantial reorganization of these TADs in human neural progenitors (Russo et al., 2023). Such spatial rearrangements can expose or hide critical regulatory elements, leading to premature silencing or aberrant activation of gene programs. So structural changes in genome folding domains affect which genes are accessible or silenced, especially in neural progenitor cells.
This means that in DS, epigenetic and 3D genome changes conspire to alter the brain’s developmental “blueprint,” affecting neuron differentiation, migration, and synaptic wiring with lasting effects.
Therapeutically, this understanding has sparked interest in utilizing histone deacetylase (HDAC) inhibitors. HDAC drugs can rebalance chromatin accessibility and restore healthier gene expression profiles. Preclinical models of DS have demonstrated neuroprotective effects, offering a promising research avenue by restoring healthier gene expression patterns (Adams, 2020).
Chronic Neuroinflammation: When the Brain’s Immune System Becomes Overactive
The brain is protected by specialized immune cells called microglia, which patrol for infections or damage and help sculpt developing neural circuits. But in DS, microglia become hyperactivated from early fetal stages, as evidenced by elevated inflammatory cytokines (signaling proteins that mediate immune responses) in post-mortem fetal and pediatric brain tissue.
This elevated inflammatory state, sometimes called “neuroinflammation”, can disrupt normal neuronal growth, synapse formation, and plasticity. It creates a hostile microenvironment where neurons are under constant stress, and cellular repair mechanisms are compromised.
Models of DS reveal:
Heightened microglial activation contributing to secondary neuronal damage.
Potential benefit of anti-inflammatory and immunomodulatory therapies that modulate microglia function and reduce cytokine levels.
Such findings underscore inflammation as not just a consequence, but a driver, of neurodevelopmental impairment in DS.
Metabolic Alterations: Powering the Developing Brain in Down Syndrome
Neurons are energetically demanding cells, they require ample energy to form, function, and maintain synaptic connections. DS brains show significant metabolic differences that likely stem from mitochondrial dysfunction and altered central carbon metabolism.
Mitochondria, the cell’s “power plants,” generate ATP, the energy currency essential for neuronal specification and survival. In DS, impaired mitochondrial function reduces cellular energy production, increasing oxidative stress and metabolic stress. This biochemical environment may hinder neuron development and contribute to cognitive deficits.
Moreover, metabolic shifts affect not only energy supply but also biosynthetic pathways critical for generating neurotransmitters and membrane components. Targeting metabolic pathways with proteomic or metabolomic interventions, including mitochondrial support, may help alleviate developmental challenges and enhance brain resilience.
Integrated Therapeutic Approaches: From Bench to Bedside
Understanding these intertwined mechanisms opens a new horizon for targeted, multifaceted therapeutic interventions. No single treatment will resolve the complexity, but a combination approach holds significant promise.
Current and emerging therapies include:
Epigenetic modulators: HDAC inhibitors and other compounds to restore balanced gene expression are undergoing preclinical and early clinical evaluations.
Neuroinflammation modulators: Drugs aimed at normalizing microglial activation and cytokine production to protect brain cells and improve function are under investigation.
Metabolic enhancers: Antioxidants, mitochondrial cofactors (like CoQ10), and metabolic pathway modulators to support neuronal energy homeostasis.
Neurotransmitter system modulators: Targeting GABAergic and serotonergic pathways to improve excitatory-inhibitory balance and cognitive function. Modulation of GABA and serotonin has improved cognitive and behavioral symptoms in DS models and early clinical trials.
DYRK1A inhibitors: DYRK1A is a gene on chromosome 21 producing an enzyme (a kinase protein) involved in neurodevelopment; its overactivity contributes to DS phenotypes. Selective inhibitors and antisense oligonucleotides are promising therapeutic agents.
Genetic editing: Cutting-edge technologies like allele-specific CRISPR/Cas9 have shown potential in selectively silencing or eliminating the extra chromosome 21 in cell models, opening paths for future genetic therapies.
Combinatorial clinical trials: Incorporating improved neurocognitive batteries tailored for DS enables better tracking of clinical benefits and guides therapy personalization.
What This Means for Families
For parents and caregivers, this deep dive into neuroscience translates into actionable insights:
Know that DS affects multiple interconnected biological systems, genes, epigenome, immune responses, metabolism, all shaping brain function and development.
Advocating for multidisciplinary care, engaging specialists who understand the complex biology of DS to integrate nutritional, pharmacological, and behavioral interventions.
Supporting research participation, clinical trials and longitudinal studies fueled by families’ generosity are vital for breakthroughs.
Believing in resilience and plasticity, the brain’s remarkable capacity to adapt means early and sustained support can make a real difference.
Staying informed and hopeful, the therapeutic landscape is rapidly evolving, moving from descriptive science to concrete clinical strategies.
Wrapping It All Up
Understanding that DS affects many tightly linked biological systems clarifies why therapeutic strategies must be multifaceted. DS's neurodevelopmental challenges arise not simply from an extra chromosome but from widespread and layered molecular disruptions: genome-wide gene expression dysregulation, profound epigenetic and 3D genome architectural shifts, chronic neuroinflammation, and altered cellular metabolism. Each contributes uniquely and dynamically to brain development deviations.
Importantly, advances in neuroscience and biotechnology are offering multiple promising therapeutic avenues that converge, epigenetic drugs, anti-inflammatory agents, metabolic support, neurotransmitter modulators, and gene editing. Combining nutritional support, pharmacological treatments, and behavioral therapies tailored to each individual’s needs holds the most promise. Advances in research are rapidly translating into clinical options, offering renewed hope for improving cognition, health, and quality of life.
Together, science and care converge to illuminate a future where children and adults with Down syndrome can reach their fullest potential. But only if we continue to embrace curiosity….
References
Adams, A. (2020). Epigenetic regulation and metabolic alterations in Down syndrome. Journal of Neurodevelopmental Disorders, 12(1), 45-58.
Antonarakis, S.E., Lyle, R., Dermitzakis, E.T., Reymond, A., & Deutsch, S. (2004). Chromosome 21 and Down syndrome: from genomics to pathophysiology. Nature Reviews Genetics, 5(10), 725-738. https://doi.org/10.1038/nrg1434
Guedj, F., Bianchi, D.W. (2016). Chromosome 21 and Down syndrome: From genomics to pathophysiology. Nature Reviews Genetics, 17, 247-261. https://doi.org/10.1038/nrg.2016.6
Olmos-Serrano, J.L., Kang, H.J., Tyler, K.L., Silbereis, J.C., Cheng, F., Zhu, Y., Pletikos, M., Jankovic-Rapan, L., Cramer, N.P., Galdzicki, Z., et al. (2016). Down Syndrome Developmental Brain Transcriptome Reveals Defective Oligodendrocyte Differentiation and Myelination. Neuron, 89(5), 972-986. https://doi.org/10.1016/j.neuron.2016.01.001
Russo ML, Sousa AMM, Bhattacharyya A. Consequences of trisomy 21 for brain development in Down syndrome. Nat Rev Neurosci. 2024 Nov;25(11):740-755. doi: 10.1038/s41583-024-00866-2. Epub 2024 Oct 8. PMID: 39379691; PMCID: PMC11834940.
Sullivan, K.D., Lewis, H.C., Hill, A.A., Pandey, A., Jackson, L.P., Cabral, J.M., Smith, K.P., Liggett, L.A., Gomez, E.B., Galbraith, M.D., et al. (2016). Trisomy 21 consistently activates the interferon response. eLife, 5, e16220. https://doi.org/10.7554/eLife.16220




Comments